Q1: Are you manufacturer or trading company?
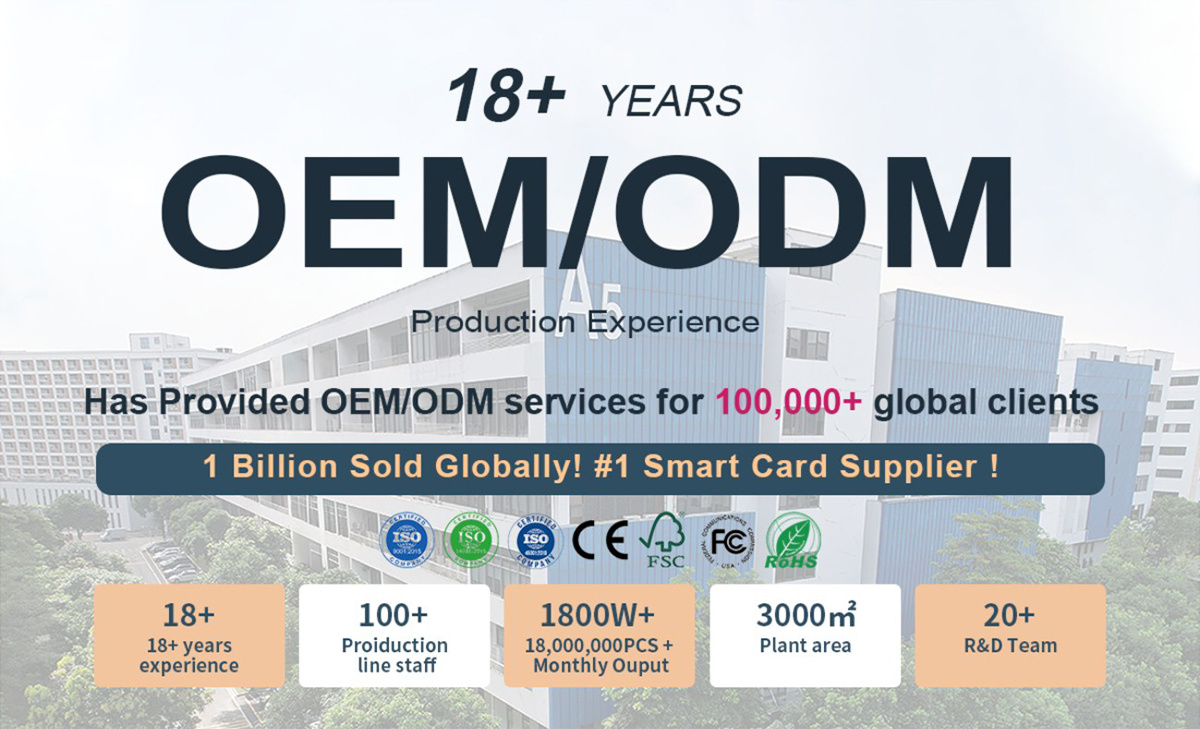
A1: We are a manufacturer specializing in high-quality RFID Medical Tags. Our main products include disposable/sterile reusable tags, patient wristbands, medical device tags and pharmaceutical packaging labels, popular globally. Compliant with ISO 13485, they feature medical-grade safety, sterilization resistance (EO, autoclave, UV), stable signals, data security and system compatibility, widely used in patient tracking, device management, pharmaceutical traceability and asset management.
Q2: Can we have our own LOGO on products and all packages?
A2: Yes, we offer comprehensive private label services for RFID Medical Tags. Customizations cover chip type (HF/UHF, ISO 15693/14443), size/shape, medical-grade materials, surface treatment, printing (LOGO, barcodes, compliance labels), functions and packaging (sterile, ESD, moisture-proof). We provide samples for confirmation after you share detailed requirements (scenario, sterilization method, compliance needs) to meet medical regulatory standards.
Q3: What is the warranty about your product?
A3: Reusable RFID Medical Tags have a 5-year warranty; disposable ones are warranted for pre-use integrity. Made of ISO 13485/FDA-certified materials and reliable chips, they ensure stable performance after repeated sterilization. For quality issues caused by us (unreadable data, chip failure, material defects, etc.) within the period, we cover shipping and offer free replacements.
Q4: Do you have quality control in your factory?
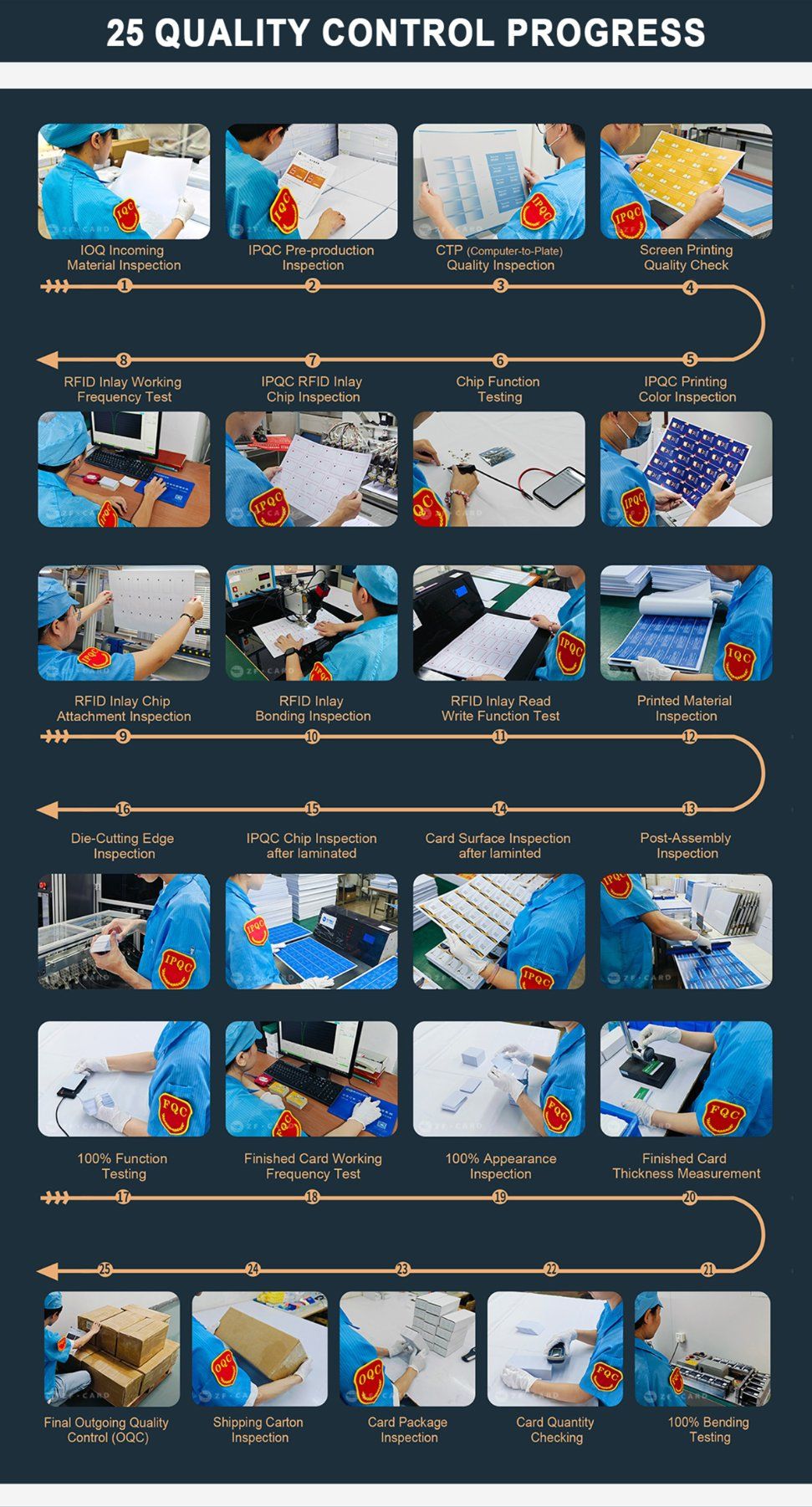
A4: Absolutely. Quality is paramount for medical tags. We conduct strict inspections throughout: material checks (sterility, hypoallergenicity, chip performance), production checks (molding, encapsulation, printing accuracy) and finished product tests (appearance, signal stability, sterilization resistance, biocompatibility) to ensure compliance and safety.
Q5: What certification does your product have?
A5: We hold authoritative certifications including ISO 13485, FDA, CE Medical, RoHS, FCC and ISO 9001. These guarantee our tags meet global medical safety, environmental and quality standards, suitable for hospitals, medical device firms, pharmaceutical companies and blood banks worldwide.
Q6: How long is your delivery time?
A6: The delivery time is 5-7 days, including customized RFID Medical Tags (chip, LOGO, size, sterilization treatment, etc.) with confirmed requirements. This efficient cycle meets urgent needs like hospital system deployment, sterilization management upgrades and epidemic prevention tracking.